
Some of these people just develop fulminant progression. Langer, MD: I think it’s the nature of the population.
#KEYNOTE 407 DRIVER#
You would think if you were greater than 50%, using the oncogenic driver paradigm, does it really matter if you get a TKI in the EGFR setting in the first or second line, as long as you get it at some point? The suggestion from KEYNOTE-024 was that even if you were highly positive and got pembrolizumab in the second line, the survival didn’t catch up, right? So I don’t know what that tells us but….Ĭorey J.

Socinski, MD: But Dr Julie Brahmer presented some of that crossover data. It may not be pembrolizumab on trial, necessarily, but it’s usually some sort of immunotherapy. When you look at those who are eligible for crossover in these trials, it’s on the order of 60% to 70%. When you actually look in the intent-to-treat population, it’s about 50%. Socinski, MD: Remind me how many people actually have crossed over?Ĭorey J. Wakelee, MD: Yes, that’s an important point. And despite that, and including this population, we still sought a consistent overall survival advantage.
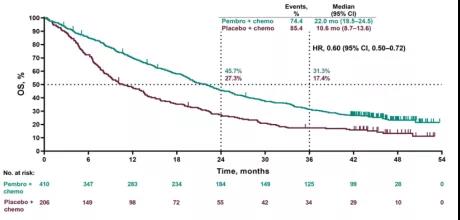
#KEYNOTE 407 TRIAL#
Patients on the control were eligible, and it was built into the trial to get single-agent pembrolizumab. All of these trials featured a crossover in the control arm at the time of progression. I want to emphasize one thing that we have not mentioned. So the fact that one trial exclusively used nab-paclitaxel, or at least in that cohort that was compared, and this one with a taxane, it may be less of a difference. And again, it may be a superior squamous drug. So that may actually be the preferential regimen to build upon. Langer, MD: If you go to the KEYNOTE-407 trial, and I believe this was in the forest plots, when they actually combined, they looked at the hazard ratios with nab-paclitaxel as opposed to solvent-based, it was actually a bit lower. Is that part of it? I don’t necessarily think so, because of other things that we’ve seen with the performance of pembrolizumab-based regimens versus other immunotherapy-based regimens.Ĭorey J. There’s that signal, potentially, of increased efficacy with nab-paclitaxel.

The performance of the control arm does matter. But again, I think your point is a good one. So each study has to be interpreted really within the context of what happened in that study. These are cross-trial comparisons that are there. There is some suggestive data that nab-paclitaxel may have more activity in squamous disease, so maybe that’s the explanation, although I don’t necessarily believe that. Obviously, it’s harder to show a difference if your control arm does better. And then on IMpower131, where the control arm was all nab-paclitaxel, the median survival was 14 months. The control arm on KEYNOTE-407, where 60% of the patients got carboplatin/taxane and 40% got carboplatin/nab-paclitaxel, is 11 months. So one of the things that bothered me about those 2 trials is the performance of the control arm. It was very difficult to make heads or tails. There is PFS, but no OS.Īnd then, at least in the initial look at the data, there was this sort of squirrely middle section for PD-L1-positive patients, intermediate, where they seemed to do worse when you added atezolizumab. And this is where, in part, IMpower131 has failed. The OS benefit really has been quite powerful. It was very similar to what we saw with KEYNOTE-189. It was positive by PFS across all PD-L1 cut points. And the randomization was to pembrolizumab with this followed by maintenance pembrolizumab or placebo. It was investigator’s choice, as to whether patients received paclitaxel or nab-paclitaxel. This was a randomized phase III trial of a backbone of carboplatin and taxane. That was in the Clinical Science Symposium, so it made it very difficult to catch both.īut KEYNOTE-407 really is similar to KEYNOTE-189. I think in KEYNOTE-407, in particular, the secondary look at the OS came out later. It was a very interesting ASCO last year, where they presented on this in 2 different sessions.

Socinski, MD: We have a little bit of conflicting data with IMpower131, which is squamous, which was kind of positive but not fully positive. Socinski, MD: Paul, we’ve also seen the data from KEYNOTE-407 in squamous disease.


 0 kommentar(er)
0 kommentar(er)
